
Fine chemicals
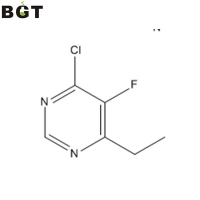 Banff Green Technologies, Inc. 4-chloro-6-ethyl-5-fluoropyrimidine,CAS 137234-74-3 查看该公司的所有供应单 联系我 报告可疑活动
产品信息:
What is 4-chloro-6-ethyl-5-fluoropyrimidine? 4-chloro-6-ethyl-5-fluoropyrimidine is a building block and pharmaceutical intermediate for synthetizing bio-active compounds such as broad-spectrum triazole antifungal agents, specifically for producing Voriconazole.
Brief introduction of Voriconazole
Application of 4-chloro-6-ethyl-5-fluoropyrimidine for synthetizing Voriconazole
Preparation of 4-chloro-6-ethyl-5-fluoropyrimidine Dissolve 80 g of 6-ethyl~5-fluoro-4-hydroxypyrimidine in 240 ml of dichloromethane to get a solution. Add 78.24 ml of triethylamine to the solution and slowly add 57.4 ml of phosphorus oxychloride thereto for more than 30 minutes. After refluxing the resulting solution for 5 hours, the reaction is completed. Cool the solution to room temperature. After that, add 352 ml of 3N HCl to the solution. The temperature should be maintained at below 200 degrees . Extract the resulting liquid mixture with 100 ml of dichloromethane. Wash the organic layer with 100 ml of water, dry it over magnesium sulfate, and concentrate it under a reduced pressure to obtain the title compound in the form of oil. There is 85.9 g of the product and the yield is 95%.
Storage of 4-chloro-6-ethyl-5-fluoropyrimidine: Store under room temperature.
联系我
与我的联系人共享
4-Chloro-6-ethyl-5-fluoropyrimidine MSDS.pdf
Download
供应单标签
137234-74-3, 4-chloro-6-ethyl-5-fluoropyrimidine, Voriconazole intermediate
|










