世界制药原料展 (CPhI Worldwide)
产品信息:
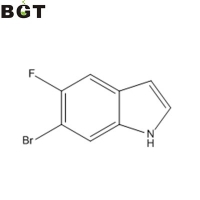
What is 6-Bromo-5-fluoro-1H-indole?
Application of 6-bromo-5-fluoro-1H-indole
The way to synthesize 6-Bromo-5-fluoro-1H-indole 1.N,N-dimethylformamide dimethylacctal (8.5ml,60mmol) was added to a stirred solution of 4-bromo-5-fluoro-2-nitrotoluene (11.8g,50mmol)in DMF (30ml) in one portion under argon at -25°,then heated at 120°for 16 hours and evaporated in a vacuum. The residual oil crystallized from MeOH/CH2Cl2(4:1) to give a purple solid (4.5g) which was dissolved in MeOH/THF (1:1,30ml) ,mixed with Raney Ni (lg),cooled to 0°and N2H4H2O(0.8ml,16mmol) was added all at once. After stirring for 90 minutes, more N2H4H2O (0.8ml) was added and stirred at 0°for 30 minutes. The mixture was filtered through Celite and the filter cakes was washed with THF, The combined filtrates were evaporated in a vacuum, and the residue was purified by chromatography [SiO2 with heptane/CH2Cl2(4:1) as eluent ] to give the indole (1.7g,16%) as an off-white solid. 2. Crude [2-(4-bromo-5-fluoro-2-nitrophenyl) vinyl]-dimethy lamine and Raney Ni in THF gave after flash chromatography through a silica gel column (eluting with 10% EtOAC in hexane ) which gave the indole as a light green solid (61%). However ,when the same enamine (1.89g,4.38mmol) in EtOH (35ml) was treated with a mixture of Fe(4.42g,79mmol) in ACOH (35ml) and stirred at 90°overnight, then filtered, evaporated and the residue purified by chromatography as above, pure 6-bromo-5-fluoro-lH-indole (0.83g,89%) was obtained as a yellow solid [Patent to the Board of Trustees of the University of Illinois , WO2008/77138 2008]. It had IR (nujol) with vmax at 3395,2925,2855,1570,1469,1451,1408,1314,1145,1105,865,763 and 505 cm-1; and The1H NMR (400MHz,CDCl3) had δ at 7.85 (br s lH),7.55 (dd.lH, J=5.6 and 0.9Hz), 7.34(d,lH,J=9.0Hz),7.23 (t,lH,J=2.8Hz) and 6.45-6.51(m,lH).
联系我
与我的联系人共享
供应单标签
6-Bromo-5-fluoro-1H-indole, 259860-08-7, (6-Bromo-3-carbamoyl-5-fluoro-indol-1-yl)-acetic acid
|