
Fine chemicals
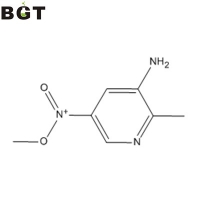 Banff Green Technologies, Inc. 2-methyl-5-nitropyridin-3-amine,CAS 143612-79-7 查看该公司的所有供应单 联系我 报告可疑活动
产品信息:
What is 2-methyl-5-nitropyridin-3-amine? 2-methyl-5-nitropyridin-3-amine is an intermediate for Flumatinib, an antineoplastic tyrosine kinase inhibitor used for the treatment of chronic myelogenous leukemia (CML).
Brief introduction of Flumatinib The chemical name of Flumatinib is 4-(4-Methyl-piperazin-1-ylmethyl)-N-[6-methyl-5-(4-pyridin-3-yl-pyrimidin-2-ylamino)-pyridin-3-yl]-3-trifluoromethyl-benzamide. Its molecular formula is C29H29F3N8O. Currently in China, it is at Phase I clinical trials for the treatment of chronic myelogenous leukemia (CML). Flumatinib is like imatinib in structure, but nonclinical studies have shown that Flumatinib was predominantly metabolized by amide bond cleavage to yield two corresponding hydrolytic products. By comparison with imatinib, the electron-withdrawing groups of trifluoromethyl and pyridine facilitated the amide bond cleavage and led to the in vivo formation of a carboxylic acid and an amine. Therefore, Flumatinib is more potent in vitro and in vivo activity than imatinib when tested against leukemia cells expressing BCR-ABL (Sun et al., 2008).
联系我
与我的联系人共享
供应单标签
51984-61-3, Flumatinib intermediate,CAS 143612-79-7
|










